Structure of hypothetical Mo-cofactor biosynthesis protein B (ST2315) from Sulfolobus tokodaii
Antonyuk, S.V., Strange, R.W., Ellis, M.J., Bessho, Y., Kuramitsu, S., Shinkai, A., Yokoyama, S., Hasnain, S.S.(2009) Acta Crystallogr Sect F Struct Biol Cryst Commun 65: 1200-1203
- PubMed: 20054111
- DOI: https://doi.org/10.1107/S1744309109043772
- Primary Citation of Related Structures:
3IWT - PubMed Abstract:
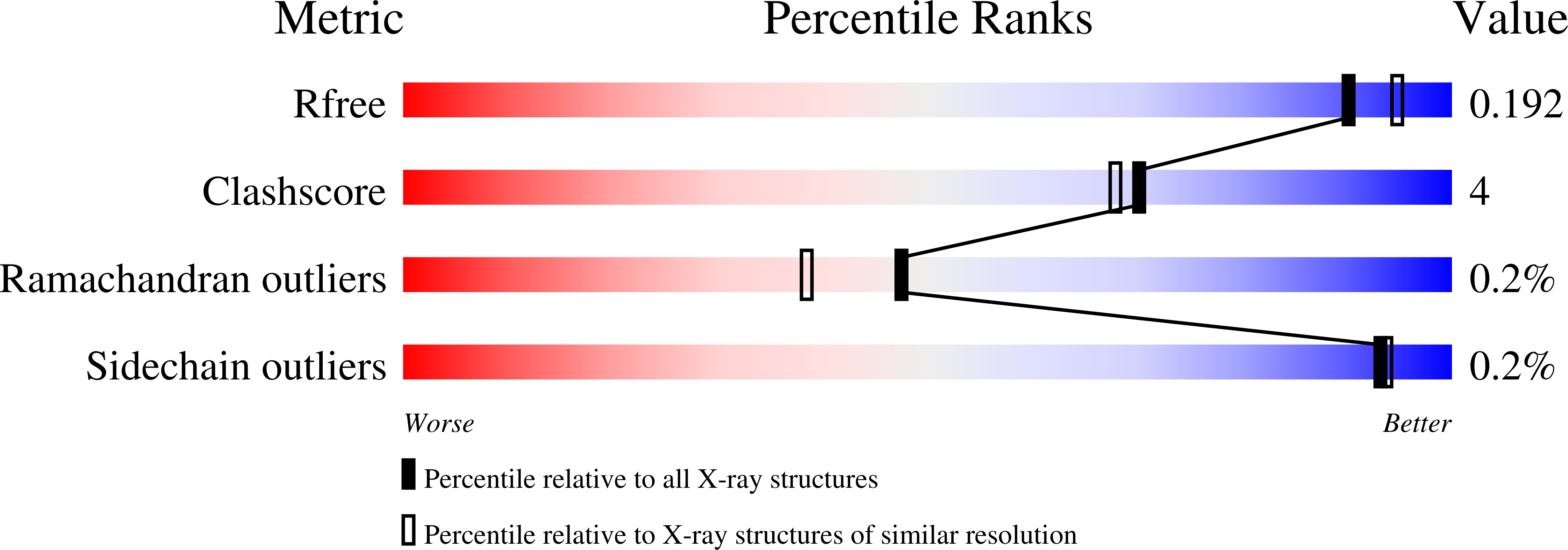
The structure of a probable Mo-cofactor biosynthesis protein B from Sulfolobus tokodaii, belonging to space group P6(4)22 with unit-cell parameters a = b = 136.68, c = 210.52 A, was solved by molecular replacement to a resolution of 1.9 A and refined to an R factor and R(free) of 16.8% and 18.5%, respectively. The asymmetric unit contains a trimer, while the biologically significant oligomer is predicted to be a hexamer by size-exclusion chromatography. The subunit structure and fold of ST2315 are similar to those of other enzymes that are known to be involved in the molybdopterin- and molybdenum cofactor-biosynthesis pathways.
Organizational Affiliation:
Molecular Biophysics Group, School of Biological Sciences, University of Liverpool, Crown Street, Liverpool L69 7ZB, England.